Abstract
BACKGROUND
In 2017, lenalidomide maintenance therapy (R-MT) was approved for patients (pts) who received an up-front autologous hematopoietic stem cell transplantation (ASCT) in the United States (US) and Europe. The total cost of care for pts on R-MT in the US has not been previously studied using a large administrative claims database. This study aimed to compare real-world outcomes and costs among pts with newly diagnosed multiple myeloma (MM) receiving R-MT vs no maintenance (No-MT) post ASCT.
METHODS
From the Truven Marketscan data set, we identified adult pts with ≥ 2 claims for MM (International Classification of Diseases, 9th/10th Revision, Clinical Modification code: 203.0x, C90.0x) 30 days apart and ≥ 1 MM treatment during the identification period (1/1/2011 to 9/30/2016). Pts were required to have continuous enrollment for 6 months pre and ≥ 6 months post initial ASCT date plus 90 days (index date), in addition to ≥ 1 full cycle of therapy with a valid first-line regimen, and evidence of ASCT during follow-up period. Patients were excluded if they had evidence of prior MM diagnosis or treatment. R-MT was defined as lenalidomide monotherapy of < 20 mg per day for ≥ 21 of 28 days. Treatment outcomes and healthcare costs during follow-up were compared among those initiating R-MT vs No-MT. Time to treatment discontinuation (TTTD) was defined as the duration from initiation of R-MT to discontinuation of R-MT. Time to next treatment (TTNT) was defined as the duration from the index date until the initiation of another second-line therapy. Kaplan-Meier (KM) and Cox proportional hazard models were performed to evaluate TTTD and TTNT. Per patient per month (PPPM) total healthcare costs were calculated at 12, 24, and 36 months among pts initiating R-MT or No-MT. Pharmacy costs included oral MM treatments and other oral medications. Outpatient costs were composed of IV medications and physician and ER visits, while hospitalizations were part of inpatient costs. Generalized linear regression models with negative binomial distribution and log link function were used to assess differences in PPPM while adjusting for differences in baseline characteristics.
RESULTS
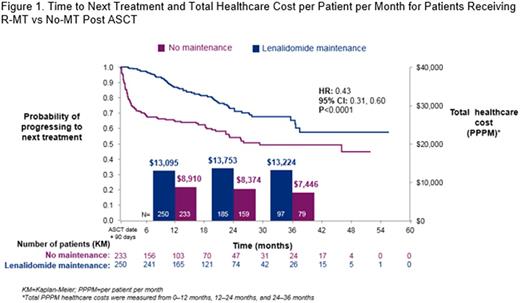
A total of 250 and 233 pts initiated R-MT and No-MT, respectively. Mean age (58.5 vs 58.0 y, P= .5236) and Charlson Comorbidity Index score (5.2 vs 5.3, P= .5234) were similar, but there were more males in the No-MT group (52.0% vs 61.4%, P= .0378). Time from start of first-line regimen to ASCT (5.48 vs 5.47 months, P= .9589) and the mean follow-up time post index date (22.1 vs 21 months, P= .1414) were similar in both groups. R-MT pts had a median duration of maintenance of 10 months. The median TTTD using the KM method was 21.02 months (95% CI: 16.78, 25.76). During the study period, a total of 60 (24%) and 99 (42.5%) R-MT and No-MT pts received a second-line treatment. At 12, 24, and 36 months, the proportion of pts who had a second-line treatment was as follows: R-MT-12.4%, 21.2%, 22.8%; No-MT-35.2%, 40.3%, 42.1%. Median TTNT was significantly longer among those treated with R-MT vs No-MT (Figure 1). Adjusted total PPPM costs during the first 12 months of follow-up was higher among pts treated with R-MT vs No-MT ($13,095 vs $8,910, adjusted difference $4,514, P < .0001). Mean pharmacy PPPM costs were higher in R-MT pts ($8,603 vs $2,269, P < .0001) while outpatient costs were higher in the No-MT group ($3,761 vs $5,360, P < .0001), and inpatient costs were similar ($731 vs $1,281, P < .5076). The adjusted difference in total PPPM costs during 12-24 months of follow-up was $6,579, P < .0001. A similar cost trend was observed in months 24-36 of follow-up; however, even fewer patients had sufficient follow-up: Adjusted difference: $6,110, P= .0024.
CONCLUSION
Pts remain on R-MT for several months to well over a year. R-MT pts incurred higher costs within the study period; however, their TTNT was significantly longer, and they were over half as likely to progress to a second-line treatment compared with No-MT pts. Pharmacy costs in R-MT pts were higher in the initial year of follow-up while No-MT pts had higher outpatient costs, presumably driven by the need for second-line therapy in a higher proportion of patients. Additional follow-up is required to assess total healthcare costs in subsequent years.
Hari: Celgene: Consultancy, Honoraria, Research Funding. Ung: Celgene Corporation: Other: Fellowship is funded by Celgene. Abouzaid: Celgene Corporation: Employment, Equity Ownership, Research Funding. Ni: Celgene: Employment. Parikh: Celgene Corporation: Employment. Agarwal: Celgene Corporation: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal